It seems like a simple question, yet how gluten digestion works is something most people would struggle to answer, including those on a gluten free diet. Gluten gets plenty of attention in terms of the foods it’s found in, how to avoid it, or the symptoms it might cause. But the conversation rarely covers what gluten is and how the body processes it.
Learning how gluten moves through the digestive system offers more than just interesting facts. It brings clarity on how the body responds to it and supports better, more informed conversations with healthcare professionals and others in the gluten free community. It also puts into perspective why enzyme-based support options like GluteGuard exist.
In this blog, we’ll start with the basics: what gluten is, how the body normally digests proteins, and why the gluten protein behaves differently in that process.
What is gluten?
Gluten is a type of protein found in grains like wheat, rye, barley, and their subspecies and hybrids including spelt, triticale, and durum wheat. It is not a single protein, but rather a combination of two protein components: glutenin and gliadin.
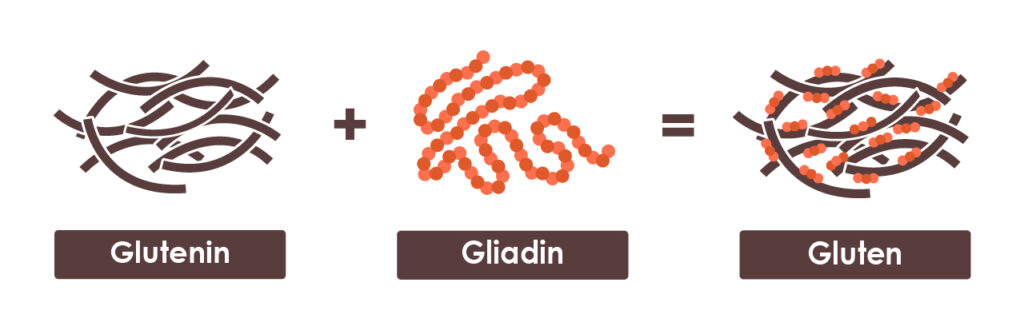
When water is added to these proteins, they bind together to form gluten – the stretchy, elastic network that gives bread, pasta, and other baked goods their familiar texture.
Unlike many other dietary proteins, gluten is more resistant to digestion by human enzymes in the gastrointestinal tract due to its unique composition.
Wait, what is protein and how is it normally digested?
To understand why gluten digestion is unique, we first need to understand how the body typically handles proteins.
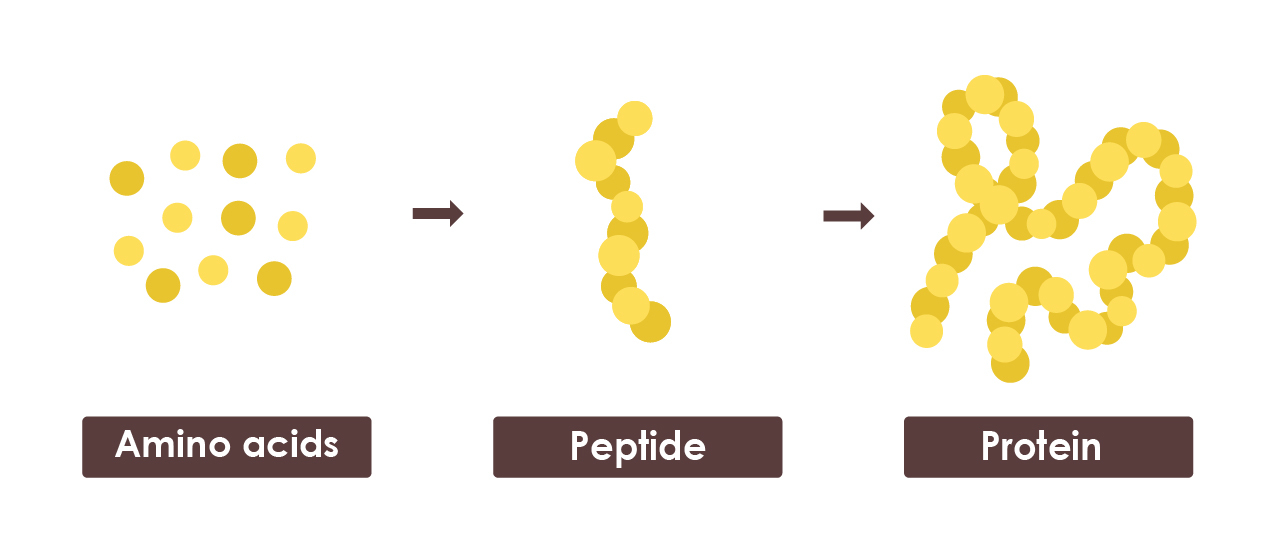
Proteins are large molecules made from smaller units called amino acids. Often described as the body’s “building blocks”, amino acids support essential functions like muscle repair, digestion and immune health.
There are 20 different types of amino acids, and the body links these together to form chains of varying lengths and sequences:
- Amino acids = single building blocks
- Peptides = short chains of amino acids (like mini proteins)
- Proteins = long chains made from many amino acids
When we eat protein, whether from animal or plant sources (such as gluten containing grains), the digestive system breaks it back down into amino acids so they can be absorbed and used by the body.
This breakdown happens in three main stages:
- In the stomach: Digestive enzymes work alongside stomach acid to cut the protein into peptides.
- In the small intestine: Additional enzymes break those peptides further into amino acids, which are then absorbed through lining of the small intestine into the bloodstream.
- Large intestine: A small fraction of proteins that remain undigested reach the large intestine, where gut bacteria ferment it into compounds like short-chain fatty acids, ammonia, and gases.
This is how the body handles most proteins in food, but gluten behaves slightly differently.
How is gluten digested in the body?
Gluten begins digestion just like other proteins, but the crucial difference occurs later in the breakdown process in the small intestine. Here’s what happens:
Step 1: Initial breakdown in the stomach
Once gluten reaches the stomach, digestive enzymes begin breaking its two main protein components, gliadin and glutenin, into long protein chains. These are then further cut into peptides, like what happens with other proteins.
Step 2: Incomplete digestion in the small intestine
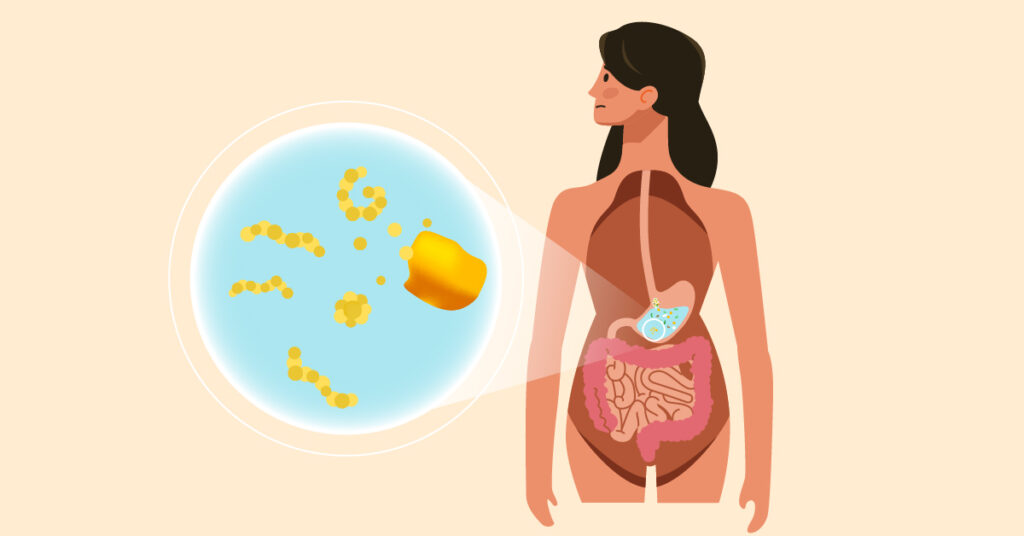
As digestion continues in the small intestine, this is where gluten’s unique behaviour becomes apparent. While most gluten peptides are broken down completely into single or small chain of amino acids, the human digestive system lacks certain enzymes needed to completely break down all gluten peptides, primarily those derived from gliadin. As a result, some peptides remain partially intact rather than being fully separated into individual amino acids.
Step 3: Interaction with the intestinal lining
These undigested gluten peptides then make their way to the lining of the small intestine, which is the main site of nutrient absorption. At this crucial point, one of two things can happen:
- In most people: the undigested gluten peptides pass through the intestinal lining and are metabolised without causing any problems.
- In people with gluten-related disorders: the body flags these undigested peptides as toxic or immunogenic. This recognition triggers a response leading to symptoms like bloating, nausea, headaches, inflammation, and in some cases, the associated autoimmune response that leads to intestinal damage.
The science behind enzyme support for gluten digestion
A strict, lifelong gluten free diet remains the only recognised treatment for managing a gluten-related disorder. Nevertheless, despite careful dietary adherence, accidental gluten ingestion is a common challenge. This can occur through unintended contamination during food preparation, incorrect labelling, social constraints, and even from unexpected sources like pharmaceuticals. It is believed that accidental gluten ingestion and ongoing consumption of trace amounts of gluten contribute to persistent symptoms, inflammation, and in some cases, intestinal damage.
This understanding of gluten’s digestive challenges is what led to the development of GluteGuard. Researchers identified that a unique enzyme called Caricain (found in the skin of unripe papaya) can break down the specific gluten peptide chains that human digestive enzymes struggle to fully digest, and that are known to trigger symptoms in gluten-related disorders.
When taken before a meal, Caricain targets these hard-to-digest gluten peptides and breaks them down into single or short chains of amino acids that can no longer trigger a reaction. By utilising this natural enzyme, GluteGuard is clinically proven to help protect against the symptoms of accidental gluten ingestion*, offering an extra layer of support in situations where gluten contamination may occur despite best efforts to maintain a strict gluten free diet.
Understanding the science behind how gluten moves through the body helps connect the dots between diet, symptoms, and management strategies for those with medically diagnosed gluten-related conditions. It shows why a strict gluten free diet is so important, and how GluteGuard can be helpful when accidental gluten is a risk. With this insight, people have the knowledge they need to make informed choices about managing their personal gluten free needs.
Sources
- University of Hawai‘i at Mānoa, (2020) Human Nutrition, Chapter 6: Protein Digestion and Absorption
- Cornell HJ, Mothes T. (1993). The activity of wheat gliadin peptides in in vitro assays for CD. Biochimica et Biophysica Acta – Molecular Basis of Disease, 1181(2), 169–173.
- Hall, N.J. et. al (2009). Systematic Review: Adherence to a Gluten-Free Diet in Adult Patients with CD. Aliment. Pharm. Ther., Vol. 30, p:315–330
- Tanner GJ (2021). Relative Rates of Gluten Digestion by Nine Commercial Dietary Digestive Supplements. Front. Nutr. Vol. 8:784850. doi: 10.3389/fnut.2021.784850
- Daveson et. al. (2020). Baseline quantitative histology in therapeutics trials reveals villus atrophy in most patients with CD who appear well controlled on gluten-free diet. GastroHep. Vol. 2, p:22–30
- Cornell JH et al. (2016). IJCD, Vol. 4, No 2 p:40-47.
*GluteGuard helps protect those with medically diagnosed gluten sensitivity from symptoms of accidental gluten ingestion. Always read the label and follow the directions for use.











