How GluteGuard Works
Protection that works before gluten can.
For smart gluten free dining, preparation is your best strategy, especially when you’re not in control of the kitchen. Here you’ll learn how GluteGuard fits into that strategy as a pre-meal tablet that steps in when gluten unexpectedly finds its way into your food.

1
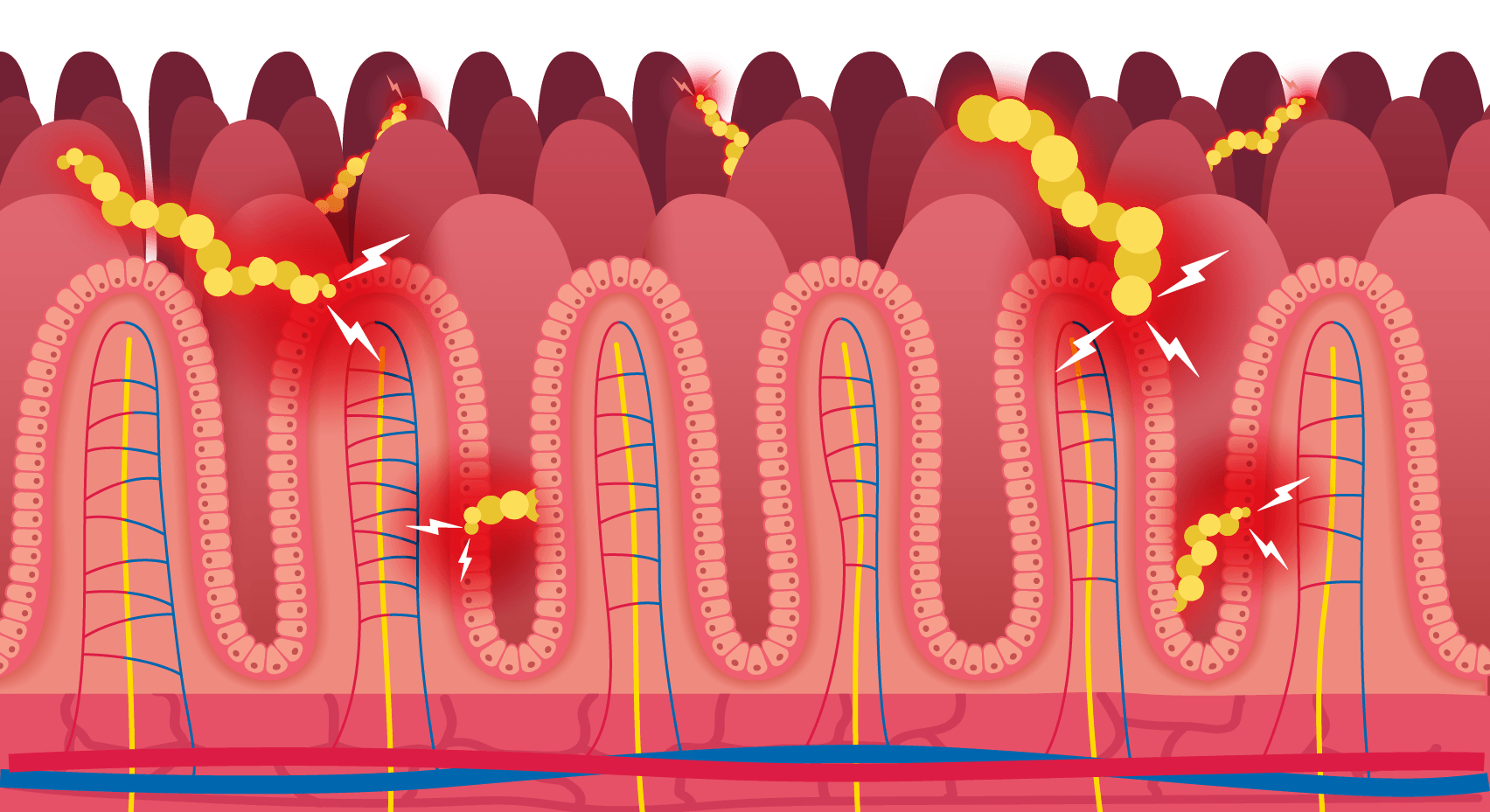
The problem with gluten? It's hard to break down.
Gluten is made up of two proteins: gliadin and glutenin. Unlike most dietary proteins that our body’s digestive enzymes break down into amino acids and small peptides for absorption, gluten’s tough structure resists complete digestion. This leaves behind larger undigested fragments called peptides.

2
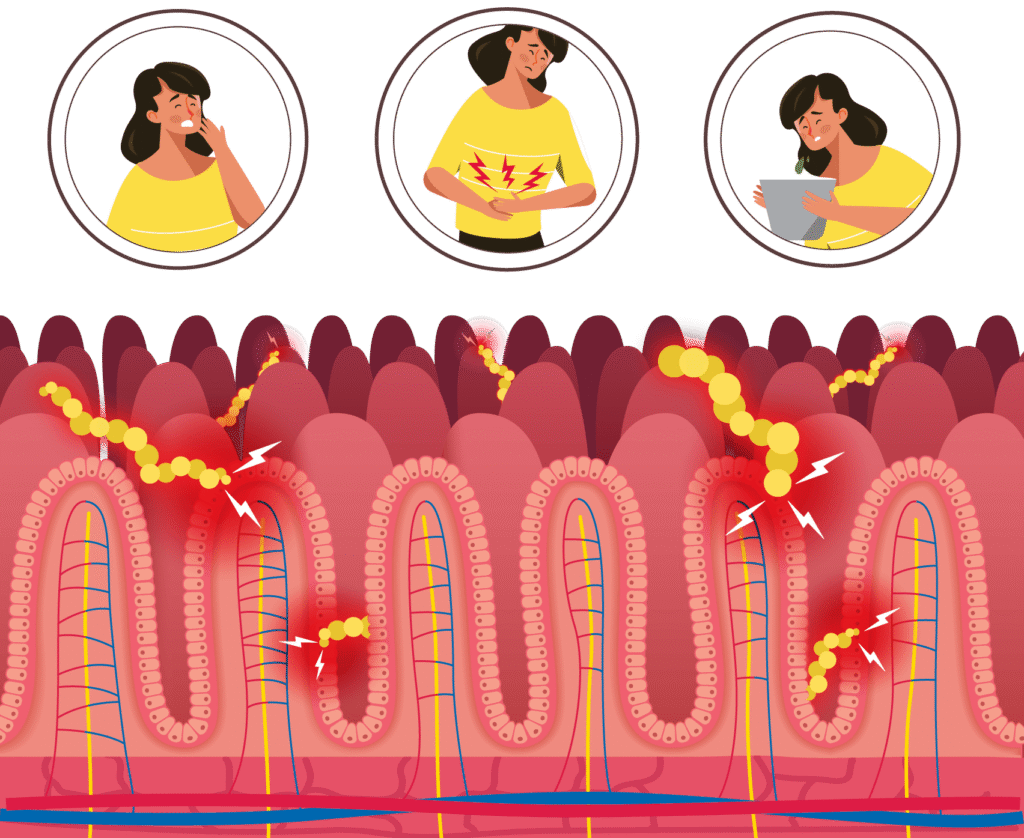
Undigested gluten peptides can trigger problems.
Most people are not affected by undigested gluten peptides. But in those with recognised gluten-related disorders, when the peptides interact with the lining of the small intestine, they can trigger a cascade of events leading to symptoms, inflammation and, in some cases, an autoimmune response that leads to intestinal damage.
3
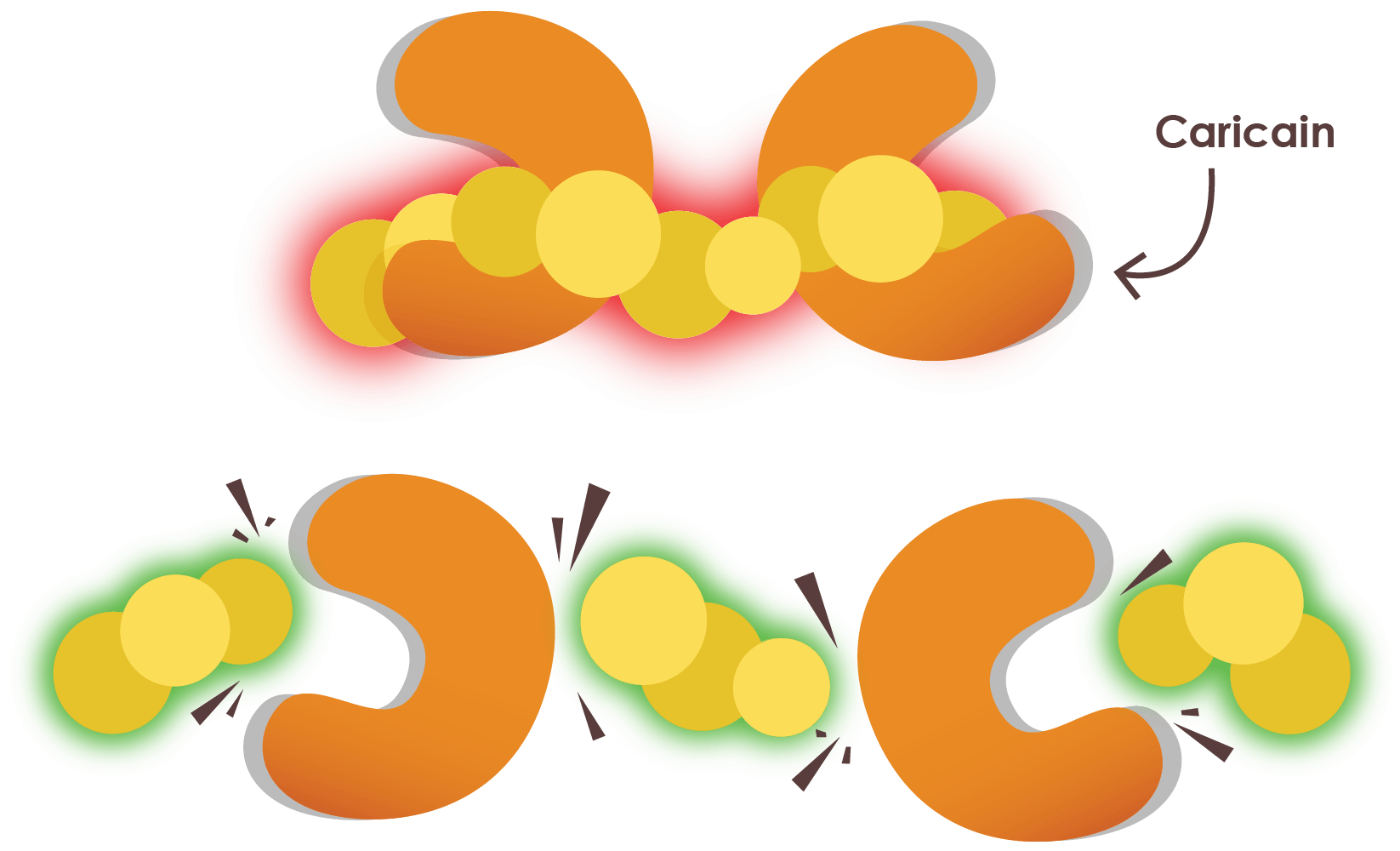
GluteGuard breaks down these peptides with Caricain.
Caricain, an enzyme found in the skin of unripe papaya, is uniquely able to target and break down these digestion-resistant gluten peptides. It breaks them into smaller fragments that can continue through the normal digestive process without triggering symptoms or immune activation.
Caricain is the powerful enzyme in GluteGuard.
4
GluteGuard delivers Caricain right where it’s needed.
When taken before a meal, GluteGuard’s enteric coating protects Caricain from the acidity of the stomach and quickly releases it in the small intestine, where it’s ready to intervene if gluten finds a way into your meal.
By taking this pre-emptive approach, GluteGuard provides protection from symptoms of accidental gluten ingestion.*
GluteGuard in action
See how GluteGuard’s enzymes get to work if gluten has snuck into your meal.
With GluteGuard
Digestion-resistant gluten peptides are broken down into smaller fragments that can pass through digestion without triggering symptoms.
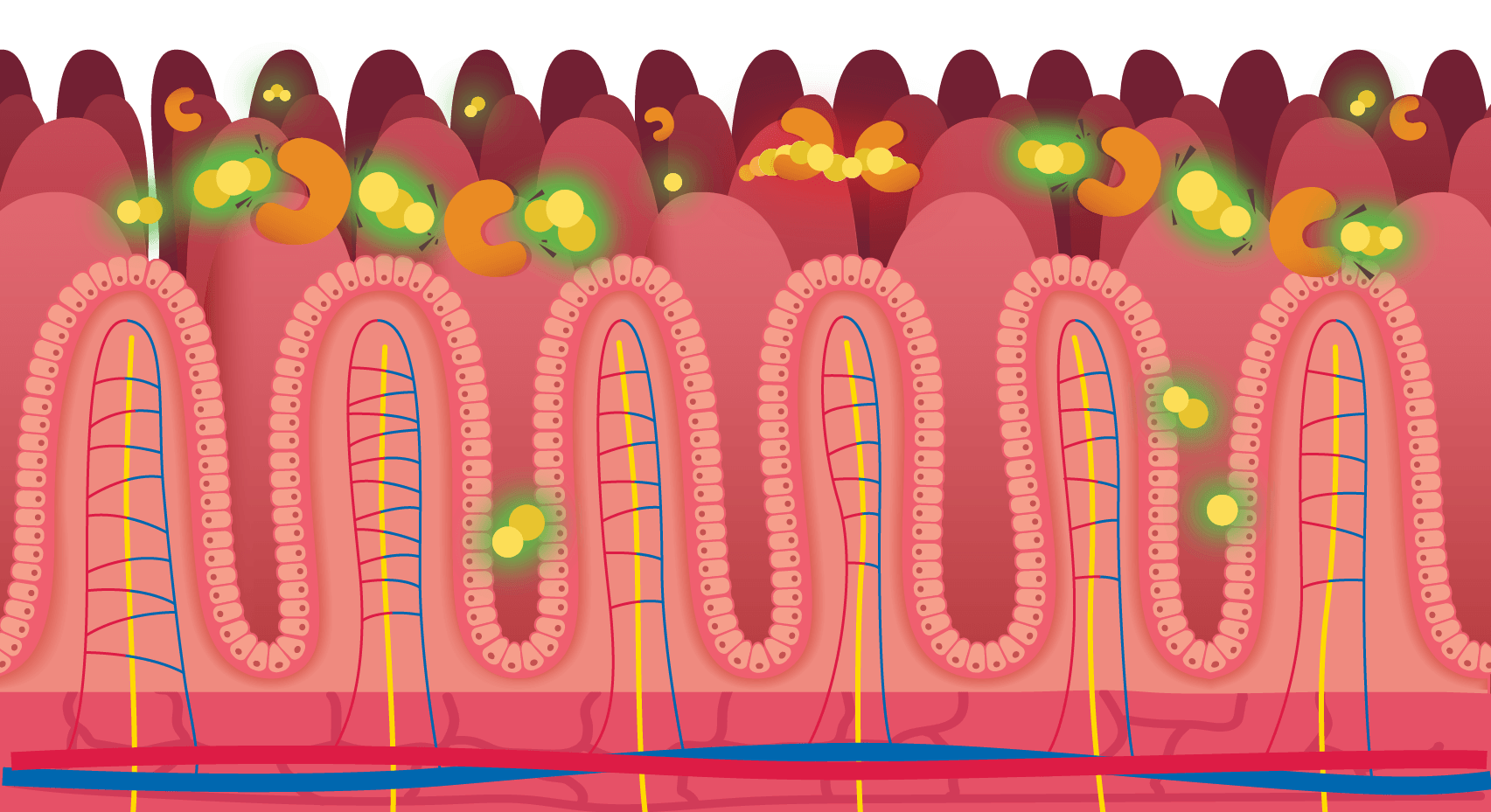
Without GluteGuard
Digestion-resistant gluten peptides remain intact and interact with the lining of the small intestine, which can trigger symptoms.
One tablet. Taken before you eat. The smart move for when gluten free isn't in your control.
Based on decades of scientific research
GluteGuard is the culmination of decades of Australian clinical and scientific research. Early work by Professor Hugh Cornell illustrating the biochemical composition of gluten has been instrumental to the understanding of gluten’s toxicity in gluten related disorders.
As a former Professor of Biological Chemistry at the RMIT University, Professor Hugh Cornell OAM, and Dr. Teodor Stelmasiak, hypothesised that if the right enzyme, of either plant or animal origin, is delivered to the gastrointestinal tract before the consumption of foods, it could complete the digestion of the toxic and immunogenic gluten peptides ultimately protecting patients against the effects caused by inadvertent gluten ingestion.
This work led to the discovery that Caricain, an enzyme extracted from the papaya fruit, was highly effective at breaking down gluten’s toxic and immunogenic peptides. This became the catalyst for the development of GluteGuard.
GluteGuard was extensively evaluated in randomised placebo controlled clinical trials exploring the effect of Caricain supplementation on symptoms and biomarkers of gluten related conditions. These trials demonstrated that GluteGuard was successful at protecting participants against the effects of a daily gluten challenge**.
For healthcare professionals enquiring about GluteGuard’s clinical research, please visit:
*GluteGuard helps protect those with medically diagnosed gluten sensitivities from symptoms of accidental gluten ingestion.
**Cornell JH et al. 2016 IJCD Vol. 4, No: 2 p:40-47, Zebrowska A et al. 2014 – IJCD Vol 2, No 2 p:58-63. Clinical studies funded by Glutagen Pty Ltd.

